Pitting corrosion affects metals and alloys such as steel, iron, aluminium and more. It is usually constrained to specific areas. It penetrates and attacks rapidly and is difficult to detect. It most commonly occurs where the passive coating layer is physically damaged or chemically attacked. This creates a weak point where water or corrosive solutions attack the substrate.
Adjacent materials will often appear unaffected. If left unchecked pitting corrosion can be devastating for roof systems or any metal structure. It occurs quickly and can easily be overlooked, which is why many consider it the most dangerous form of corrosion.
Pitting Corrosion Explained
Pitting corrosion is a cavity, hole or pit that forms in a small area or point. The pits or holes are obscured by a small amount of corrosion product (rust) on the surface. When a cathodic reaction in a large area (coating) sustains an anodic reaction in a small area (exposed metal), a pit, cavity or small hole will form. Oxidation occurs in the metal even when there is no supply of oxygen.
High electron demand by the large cathode is put on the small anode, the result is intense pitting corrosion. It will be subtle and happen rapidly with very harmful effects. Only a small spot of rust is visible on the surface while damage happens deep in the metal structure below.

What Causes Pitting Corrosion?
Pitting corrosion occurs when the cathode (damaged coating) is large and the anode (exposed metal) is small. Typically the surface protection layer or film becomes the cathode when it is damaged and cracked. A small area of metal is then exposed and becomes the anodic.
Pitting is vigorous when the solution on the metal surface contains chloride, hypochlorite or bromide ions. Other harmful solutions are those that contain fluorides and iodides. Sulphides and water are also known to enhance the pitting process.
The most common pitting corrosion causes are;
- Cracks in protective coating
- Scratches, scuffs & small chips
- Non-uniform stress
- Defective metal substrate
- Turbulent fluid flow
- Non-uniform protective coating
- Chemical attack on protective coating
Metals prone to pitting corrosion are;
- Stainless steel
- Chromium
- Passive iron
- Mercury
- Cobalt
- Aluminum
- Copper
- Associated alloys
Another example of pitting corrosion occurs when a metal is poorly maintained and exposed to water droplets and dust particles. The area below the droplet is insufficiency oxygenated while the surrounding areas are well oxygenated. This results in differential aeration corrosion where surrounding areas are cathodic and the small area below the droplets and dust particles become anodic. Electrons flow through the metal and are met by water and oxygen. Ions are formed and defuse together to produce rust. Pits, cracks and crevices develop in the metal as the rust is produced.
Types of Pitting Corrosion
Pitting corrosion can appear in a variety shapes. The shape of the pit depends largely on the material affected and the direction of the grain within that material. Passive metals and alloys are most commonly affected, these include stainless steel and aluminium. However almost any metal or material susceptible to corrosion can be affected.
Trough Pitting Corrosion
Trough pit shapes tend to be hemispherical, cup-shaped or irregularly shaped. Trough pitting corrosion occurs when the passive film (protective layer) is compromised and the metal wall is attacked forming narrow and deep troughs. Their flat walls expose the crystal structure of the metal. These can quickly perforate the thickness of the material, for example a roof sheet, truss or gutter component.

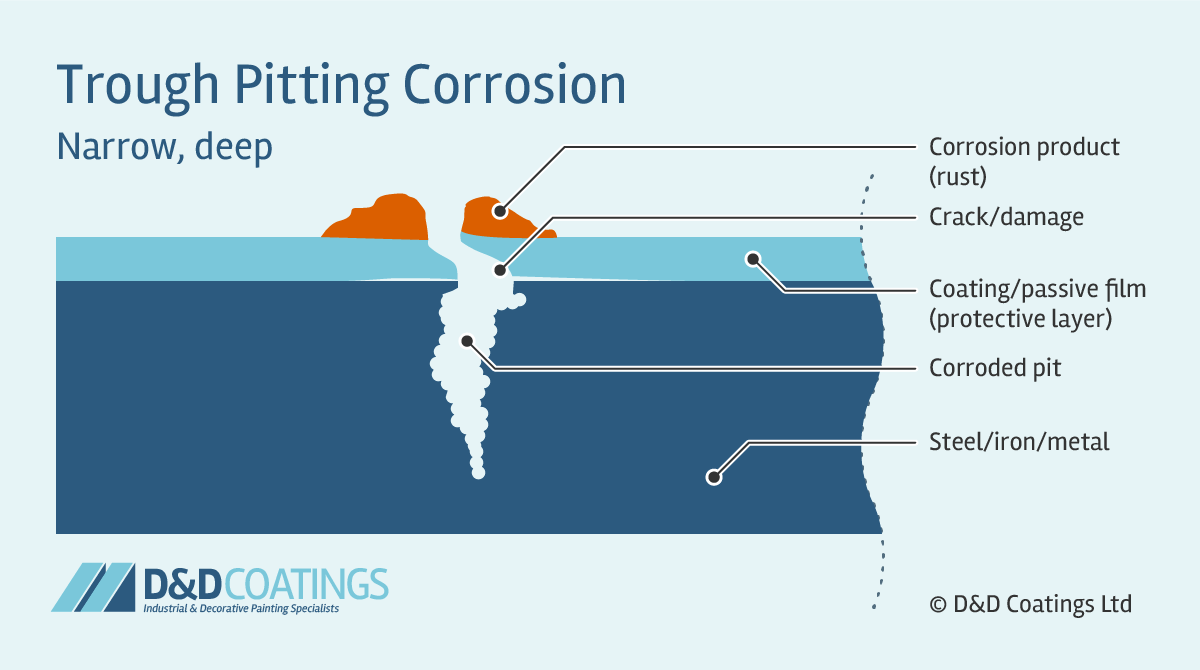

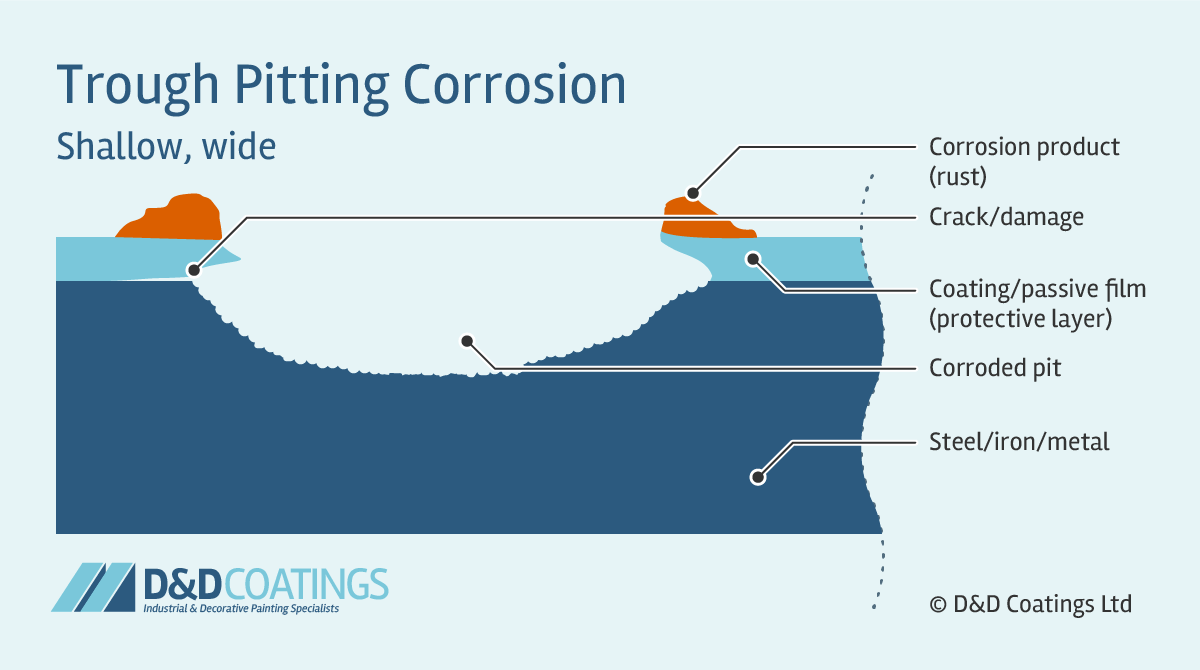
Sideway Pitting Corrosion
Sideway pits are covered with a semi-permeable membrane of corrosion product (rust) and appear in horizontal grain attack, undercutting and subsurface shapes. Sideway pitting corrosion can penetrate the metal very quickly. It is very difficult to detect because the surface of the metal will appear unaffected and free from corrosion. With only a few small spots of rust it may appear as if damage is very minor.

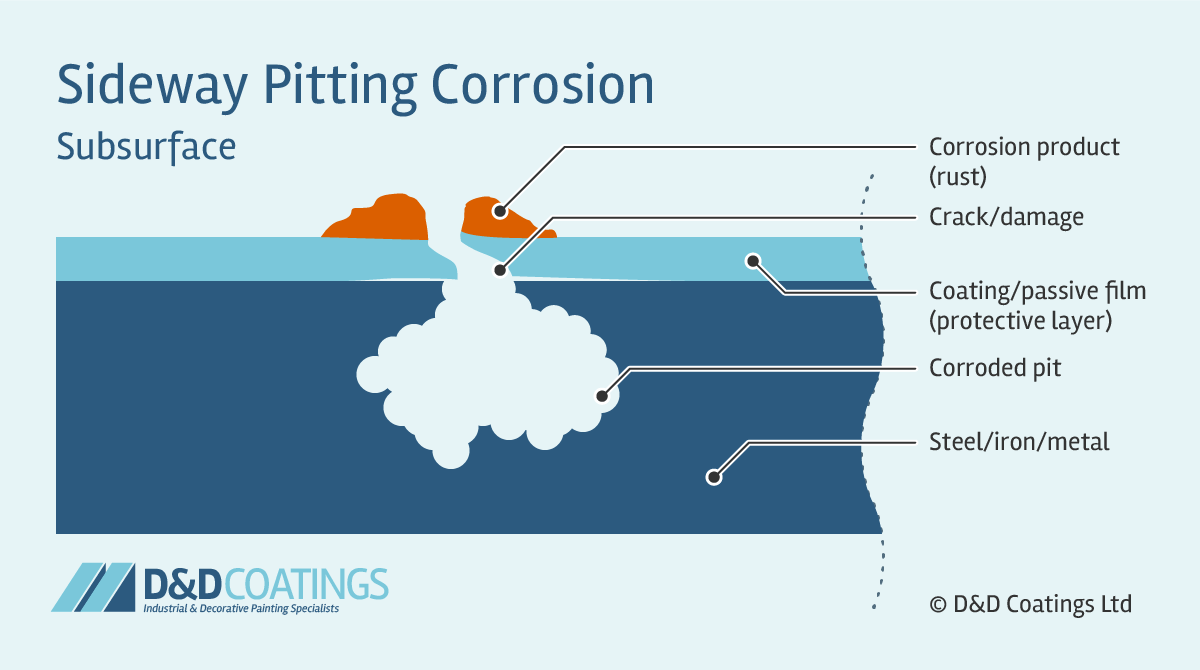
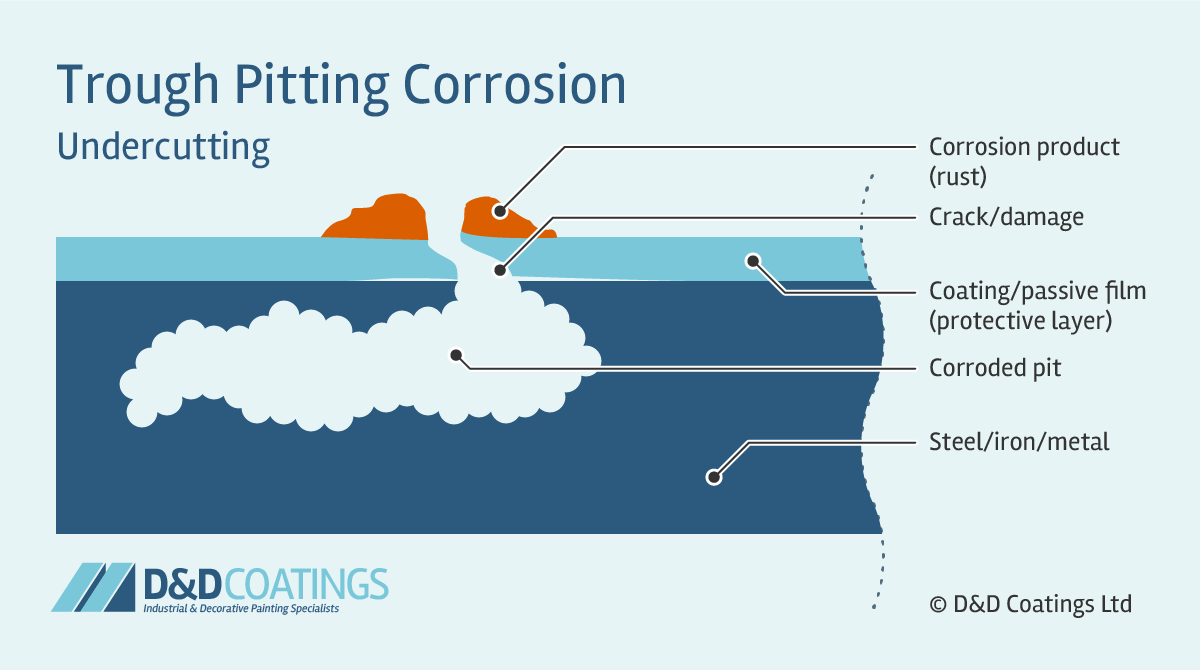
How to test for Pitting Corrosion?
A visual examination of the corroded metal surface is the first and basic method used. Count the number of pits through a microscope eyepiece over a defined surface area such as 20cm2 to determine the approximate size and distribution of the pits. The number of pits is not necessarily the most important factor to consider. Pit depth is by far the biggest danger. One narrow deep pit can be more dangerous the many shallow pits. A metallographic cross section to measure the depth of the pits will reveal the intensity of the attack.
Sonics testing is where ultrasonic pulses of sound energy are transmitted through an oil or water based couplant onto the metal surface. Waves are generated and reflect echoes that are converted in to electrical signals. These signals can be interpreted to show the location of pits, crevices and flaws in the metal. This test has good sensitivity and provides instantaneous information about the depth, width and location of the pits and flaws.
Electromagnetic testing is used to detect defects or irregularities in the structure of electrically conducting materials such as steel and iron. Materials with defects will produce a magnetic field that is different from that of a reference material without defects.
Electrochemical testing to measure pitting in any metal such as cyclic polarisation and potentiostatic tests are also an option. These are short term electrochemical tests that provide instant results.
An immersion test or weight loss method is another option. These tests take more time to run. They involve removing a metal sample and immersing in a solution. After a few days it can be removed so the corrosion rate can be calculated. You can observe the pits and pit depth under a microscope and make the necessary calculations.
How to treat Pitting Corrosion?
It is bed to use recommended cleaning procedures to expose the pits fully and remove the corrosion products. Avoid using solutions that attack the base metal excessively. It is advisable during cleaning to periodically probe the pits with a pointed tool. This will enable you to determine the extent of any undercutting or subsurface corrosion. Vigorously scrubbing the pit with a wire brush will enlarge the openings sufficiently. Removing the corrosion products and undercut metal you will help to evaluate the extent of damage.
When the metal material is clean and free from debris carry out a final inspection of the damage. If you are happy the corrosion has been caught in time you may proceed with applying a base primer coating followed by one or two more top layers. If the damage is severe and the component is too weak it will need to be replaced.
Zinc phosphate priming is on of the most popular coating methods to protect against pitting corrosion. Specially formulated primers such as zinc phosphate improve corrosion resistance.
Zinc spray metallising is a technique that is very effective against corrosion. It has smooth finish which is aesthetically appealing and popular. It doesn’t provide the same protection as hot dip galvanising but because this is a cold process there is no risk of distorting the metal. Zinc spray metallising is ideal for use on ornate metal items such as art exhibits, metal railings and fences.
Chemical coating uses electrostatic or compressed air to apply a specially formulated powdered material to a steel surface. It is then melted to form a smooth protective film. Steel treated in this way is not only protected against corrosion and UV damage, it is also highly resistant to peeling, scratching and cracking.
Hot dipping is a galvanisation method of coating that can be used on steel of all shapes and sizes. It involves immersing the steel in a bath of molten zinc at temperatures up to 450°C. Steel galvanised in this way is a particularly popular approach for pipe related applications, because its highly protected against corrosion as well as extreme weather conditions.
How to prevent and protect against Pitting Corrosion?
Environmental factors that cause pitting corrosion should be minimised, where possible. Humidity, temperature, chloride and pH acids and salt levels should be controlled and minimised.
For cathodic protection, metal that is at risk should be coated with a reactive metal that corrodes more easily. This can come in the form of a galvanised zinc coating or similar. The reactive metal will act as the anode and corrode first preventing pitting in the substrate.
For protection against chemical attack, metals resistant to corrosion such as alloys are better. Alloys that contain titanium, nitrogen, chromium and molybdenum are very effective in environments with high levels of chloride ions.
Pitting corrosion can be controlled by:
- Use of a coating that will prevent pitting on metal surfaces
- Using more corrosion resistant materials
- Ensuring that the fluids in contact with the material is washed away regularly
- Use of cathodic protection
- Avoiding stagnant zones
- Inhibitor use / fluid chemistry control
- Maintain the protective film of the material
More Reading
Pitting corrosion – Wikipedia
Examination and evaluation of pitting
Different Types of Pitting Corrosion
Guadalajara Sewer Explosion due to Pitting Corrosion



